Atlantic Optics provides innovative technology used in the diagnosis and management of glaucoma and vascular disease.

The Falck Multifunction Device is the only device cleared by the U.S. FDA for ophthalmodynamometry, tonography, serial tonometry, and dry eye testing.

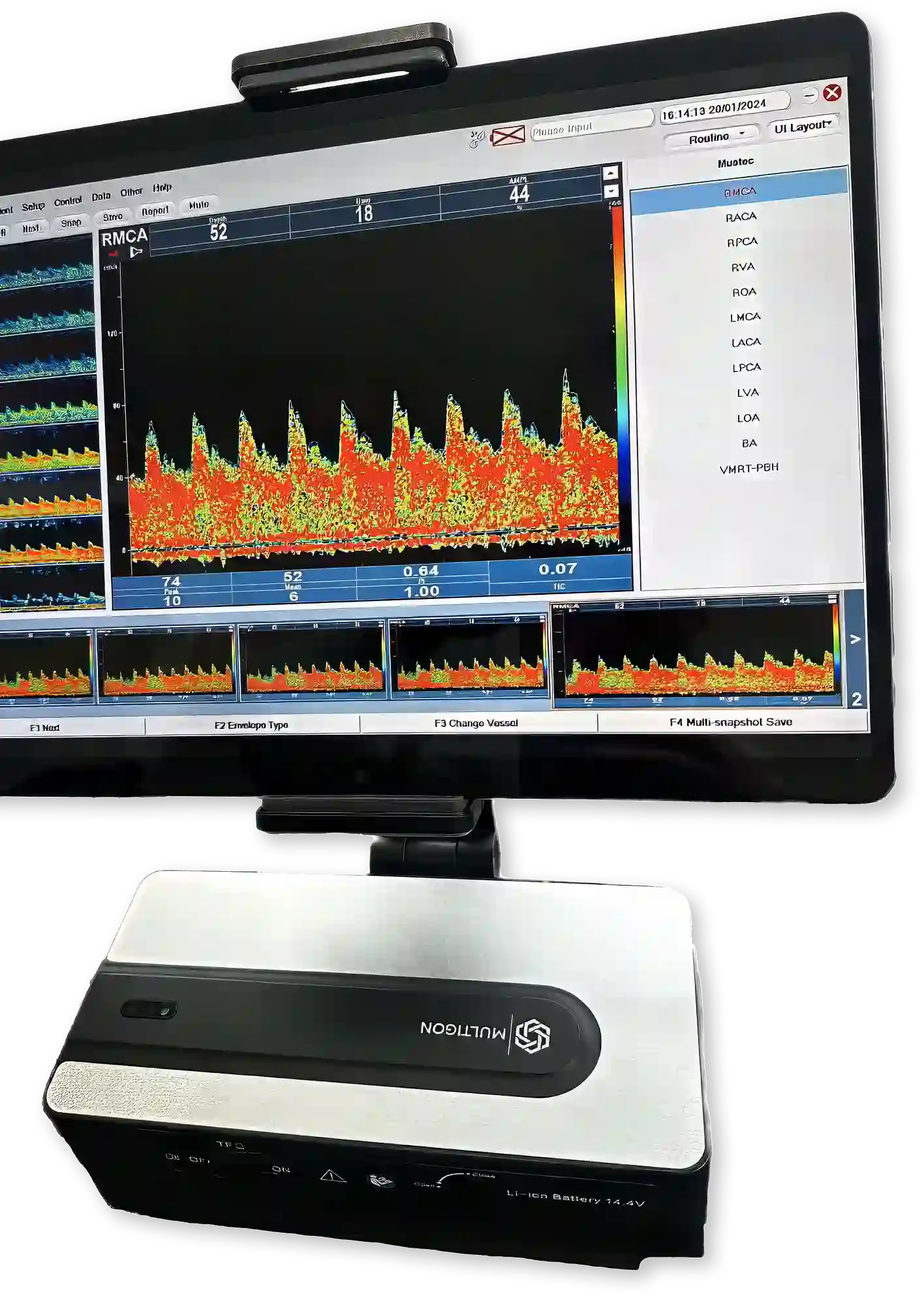
Multigon USA and Falck Medical Technologies has established a proven model of legitimate application in ophthalmology, to ensure TCD is only ordered when medically necessary. The Falck multifunction device is the only FDA cleared device that quantitates the mean central retinal artery (MCRAP) perfusion pressure or ophthalmodynamometry (ODM). A reduced MCRAP demonstrates focal ischemia which is most probably caused by proximal vascular disease. TCD is the most sensitive way to determine if stenosis exists in the large basal vessels of the Circle of Willis and the distal ICA's.
Explore our video gallery where you can learn more about the eye care market’s greatest solutions.
Falck Medical FMD Instructional Video
Proper Insertion of Fixed-Use Prism for Falck Medical FMDDevice
Introduction to the Falck Medical, Inc FMD Multifunction Device.
FMD Technology Overview
Falck Medical, Inc., FMD Serial Tonometry Measurement.
Falck Medical, Inc., FMDTonography Measurement.
Falck Medical, Inc., FMD Ophthalmodynamometry Measurement.
Falck Medical, Inc., FMD Coding and Reimbursement
Pearls for Using the FMD Device from Falck Medical Inc
Press Conference for the FMD at the Boston AAO 2021 Meeting

Devices
These devices use technologies that aid in blindness detection and stroke prevention.

Parts
Each individual prism helps protect against infectious disease transmission